28 Fda Off Label
An exception to this is the use of some controlled substances such as opioids pain medicines like morphine and fentanyl. This practice is called off label because the drug is being used in a way not described on its package insert.
 Fda Loses Another Free Speech Case Over Off Label Use Foundation
Fda Loses Another Free Speech Case Over Off Label Use Foundation
This draft guidance has been prepared by the office.

fda off label. Unapproved use of an approved drug is often called off label use. Search for fda guidance documents responding to unsolicited requests for off label information about prescription drugs and medical devices december 2011 download the draft. Off label means the medication is being used in a manner not specified in the fdas approved packaging label or insert.
This label is a written report that provides detailed instructions regarding. Is off label drug use legal. Every prescription drug marketed in the us.
The drug labels and other drug specific information on this web site represent the most recent drug listing information companies have submitted to the food and drug administration fda. Fda provided several examples of the type of information that might not appear on a products label or labeling but that could be considered cfl under the guidance including. Off label drug use refers to the practice of prescribing a drug for a different purpose than what the fda approved.
Fdas policies on unsolicited requests for off label information including those that firms may 25 encounter through emerging electronic media. Information sheet off label and investigational use of marketed drugs biologics and medical devices guidance for institutional review boards and clinical investigators january 1998. The off label use of fda approved drugs is not regulated but it is legal in the united states and many other countries.
A comparison of the safety or efficacy of a medical product for its approved indication to another medical product approved. Carries an individual fda approved label. See 21 cfr part 207 the drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor.
Off label is also called non approved or unapproved use of a drug. This term can mean that the drug is.
 Fda Off Label Hearing Day One Policy Amp Medicine
Fda Off Label Hearing Day One Policy Amp Medicine
 Off Label Drug Usage Information Sharing Platform Launched By Fda
Off Label Drug Usage Information Sharing Platform Launched By Fda
 Compendia The Bridge Between Fda Approved Indications And Off
Compendia The Bridge Between Fda Approved Indications And Off
 F D A Deal Allows Amarin To Promote Drug For Off Label Use The
F D A Deal Allows Amarin To Promote Drug For Off Label Use The
 Ketamine Fda Approval And Off Label Use Of Drugs Allison Wells
Ketamine Fda Approval And Off Label Use Of Drugs Allison Wells
 Here S How Fda Officials Think You Can Legally Promote Off Label
Here S How Fda Officials Think You Can Legally Promote Off Label
 U S Constitution Vs Fda Off Label Discussions No Longer Faux
U S Constitution Vs Fda Off Label Discussions No Longer Faux
 Off Label Use Of Atypical Antipsychotics An Update Comparative
Off Label Use Of Atypical Antipsychotics An Update Comparative
 Off Label Drug Use And Fda Review Of Supplemental Drug
Off Label Drug Use And Fda Review Of Supplemental Drug
 Women Risk Vaginal Rejuvenation Injuries With Off Label Use Of
Women Risk Vaginal Rejuvenation Injuries With Off Label Use Of
 Article Off Label Medication And Use Of Unapproved Drugs Citation Lu
Article Off Label Medication And Use Of Unapproved Drugs Citation Lu
.jpg ) Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
Off Label Prescribing Surprising Uses For 7 Fda Approved Drugs
 Free Speech Amp Off Label Branding Policybrief The Federalist
Free Speech Amp Off Label Branding Policybrief The Federalist
 Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
 Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
 Dii Pov Fda Off Label Guidance
Dii Pov Fda Off Label Guidance
 The Guide To Off Label Prescription Drugs New Uses For Fda
The Guide To Off Label Prescription Drugs New Uses For Fda
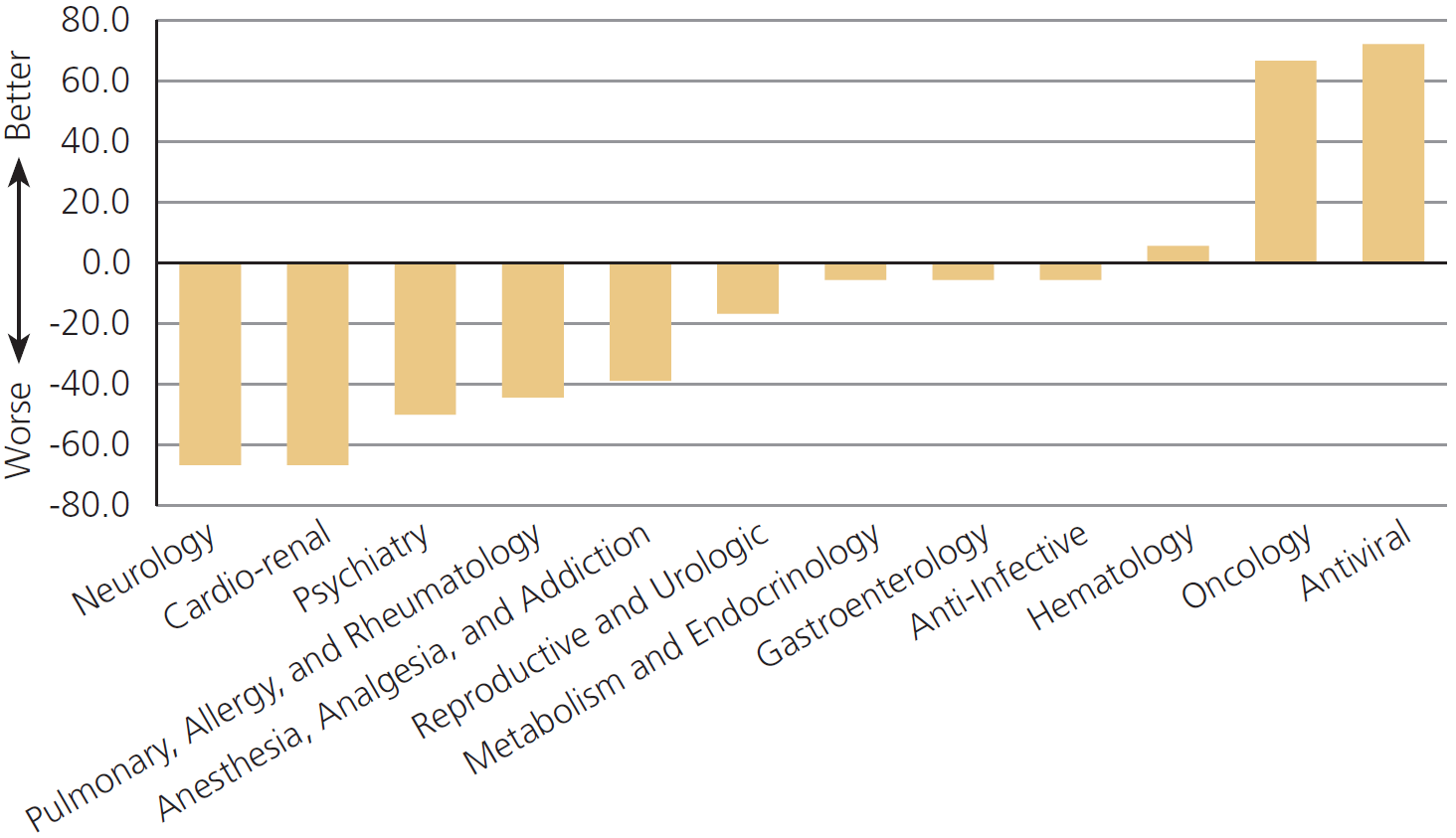
 Off Label Treatments Were Not Consistently Better Or Worse Than
Off Label Treatments Were Not Consistently Better Or Worse Than
 Fda Allows Off Label Health Care Economic Discussions Is More To
Fda Allows Off Label Health Care Economic Discussions Is More To
 First A Pricing Scandal For Catalyst S Firdapse Next Off Label
First A Pricing Scandal For Catalyst S Firdapse Next Off Label
 Off Label Non Fda Approved Indications For Topical Imiquimod
Off Label Non Fda Approved Indications For Topical Imiquimod
 Off Label Prescribing Of Antipsychotics For Youths Who Should Be
Off Label Prescribing Of Antipsychotics For Youths Who Should Be
 Fda Approved Doses For On Label Indications And Dosage Ranges
Fda Approved Doses For On Label Indications And Dosage Ranges
 Off Label Prescribing Shows That Less Regulation Means More
Off Label Prescribing Shows That Less Regulation Means More
 F D A Deal Allows Amarin To Promote Drug For Off Label Use The
F D A Deal Allows Amarin To Promote Drug For Off Label Use The
 Off Label Use Of Drugs C3ihc Drug Safety Amp Healthcare Outsourcing
Off Label Use Of Drugs C3ihc Drug Safety Amp Healthcare Outsourcing

Belum ada Komentar untuk "28 Fda Off Label"
Posting Komentar