29 Fda Off Label Promotion
State and federal legislators are prepping changes that would give wider latitude to pharmaceutical manufacturers and unravel fda rules on off label promotion according to a new editorial in plos medicine from drs. Michael sinha and aaron kesselheim of the program on regulation therapeutics and law portal division of pharmacoepidemiology and pharmacoeconomics department of medicine brigham and womens hospital and harvard medical school.
 The First Amendment Applies To The Fda Too Reason Com
The First Amendment Applies To The Fda Too Reason Com
Unapproved use of an approved drug is often called off label use.

fda off label promotion. The site is secure. Explicit off label promotion occurs when a manufacturer makes direct claims about a product to promote it for an unapproved use. As a result there have.
This is one of the more high profile and dangerous forms of pharmaceutical fraud an illegal practice that has harmed thousands of people and defrauded medicare and medicaid of billions of dollars. To involve off label promotion but in actual fact involve the permitted non promotional distribution of off label information. Off label promotion comes in two formsmarketing a device that has not received fda approval and promoting an approved device for an unapproved use.
The fda recognizes that the public health is actually served by a certain amount of manufacturer distribution of information about off label uses. Off label marketing is the promotion of a drug or medication by manufacturers for a purpose other than what the food and drug administration fda has approved. A comparison of the safety or efficacy of a medical product for its approved indication to another medical product approved.
This term can mean that the drug is. The federal food drug and cosmetic act fdca which provides the overarching framework for pharmaceutical regulation in the united states does not explicitly prohibit off label promotion but it permits fda to regulate manufacturers marketing and branding of drugs and prohibit the introduction of new unapproved drugs into the market. As attention on the off label promotion of prescription drugs intensifies it is worth examining how this evolving regulatory landscape applies to the promotion of other prescription drugs that have not been deemed safe and effective by the food and drug administration fda for the use promoted.
Off label promotion can be explicit or implicit. Fda provided several examples of the type of information that might not appear on a products label or labeling but that could be considered cfl under the guidance including. Before sharing sensitive information make sure youre on a federal government site.
1 recent changes to federal law. Used for a disease or medical condition that it is not approved to treat such as when a chemotherapy is approved to treat one type of cancer but healthcare providers use it to treat a different type of cancer. The https ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Opponents of off label promotion draw a distinction between a physicians decision to prescribe a drug for an off label use and a pharmaceutical companys promotion of that off label use. Federal government websites often end in gov or mil. They argue that promoting a drug for off label use inappropriately exposes patients to risk.
 Off Label Drug Prescription Promotion Marketing First Amendment
Off Label Drug Prescription Promotion Marketing First Amendment
 Public Citizen Fda Must Stop Manufacturers Off Label Promotion
Public Citizen Fda Must Stop Manufacturers Off Label Promotion
 Fda Allows Amarin To Market Off Label Use Of Vascepa Upi Com
Fda Allows Amarin To Market Off Label Use Of Vascepa Upi Com
 Fda Is Illegally Allowing The Off Label Promotion Of Medical
Fda Is Illegally Allowing The Off Label Promotion Of Medical
 Fda Guidance Regarding The Promotion Of Off Label Uses Of Drugs
Fda Guidance Regarding The Promotion Of Off Label Uses Of Drugs
 First Amendment Decision On Off Label Promotion Will Not Stop Fda
First Amendment Decision On Off Label Promotion Will Not Stop Fda
 Promotional Communication Keeping Up With Fda S Off Label Use
Promotional Communication Keeping Up With Fda S Off Label Use
 Fda Off Label Promotion Guidelines
Fda Off Label Promotion Guidelines
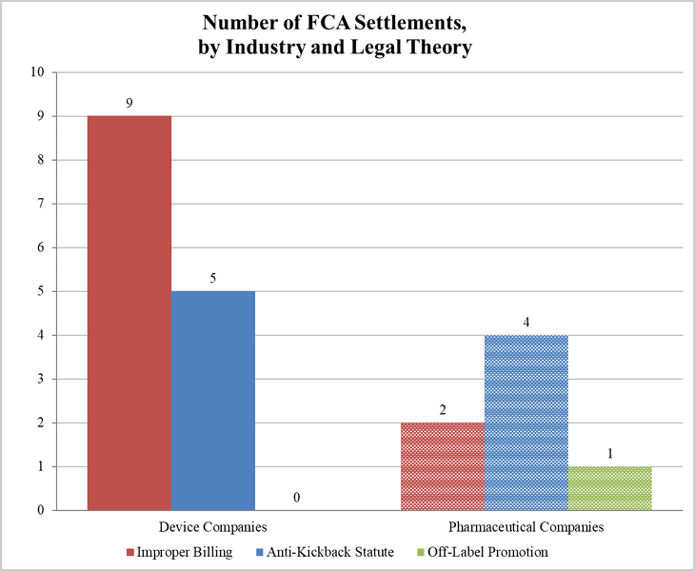 Gibson Dunn 2018 Year End Fda And Health Care Compliance And
Gibson Dunn 2018 Year End Fda And Health Care Compliance And
Pharmaceutical Industry Off Label Promotion And Self Regulation A
 Amarin V Fda And Public Meeting On Off Label Promotion Signal An
Amarin V Fda And Public Meeting On Off Label Promotion Signal An
 A Murky Future For Off Label Promotion States Vs Fda Arizona
A Murky Future For Off Label Promotion States Vs Fda Arizona
 Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
Fda Regulation Of Promotion Amp Advertising Part 6 First Amendment
 The Legalization Of Off Label Promotion Vascular Solutions Beat
The Legalization Of Off Label Promotion Vascular Solutions Beat
 Another Successful First Amendment Challenge To The Prohibition Of
Another Successful First Amendment Challenge To The Prohibition Of
 F D A Deal Allows Amarin To Promote Drug For Off Label Use The
F D A Deal Allows Amarin To Promote Drug For Off Label Use The
 Whose Right To Free Speech How Pharma Is Co Opting The First
Whose Right To Free Speech How Pharma Is Co Opting The First
 Ppt Off Label Promotion Of Medical Devices Powerpoint
Ppt Off Label Promotion Of Medical Devices Powerpoint
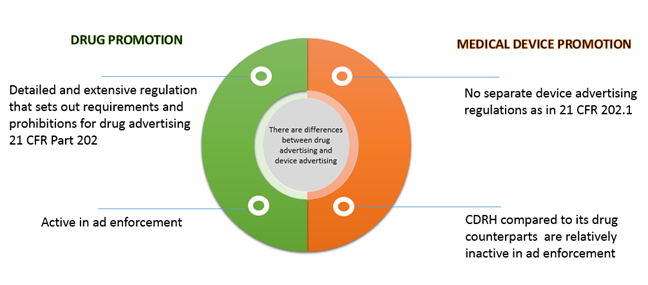 Fda Regulations On Advertising And Promotion Of Drugs And Medical
Fda Regulations On Advertising And Promotion Of Drugs And Medical
 Joseph Ross Pa Twitter Our Review Of 18 Months Of Pharmaceutical
Joseph Ross Pa Twitter Our Review Of 18 Months Of Pharmaceutical
 Pharma Marketing Blog Will Fda Cite This Vyvanse Web Image As Off
Pharma Marketing Blog Will Fda Cite This Vyvanse Web Image As Off
 Fda Issues Draft Guidances On Drug Device Labeling Fiercebiotech
Fda Issues Draft Guidances On Drug Device Labeling Fiercebiotech
 Direct To Consumer Off Label Drug Promotion Survey Results
Direct To Consumer Off Label Drug Promotion Survey Results
 Updated Off Label Promotion Are Fda S Rules About To Unravel Raps
Updated Off Label Promotion Are Fda S Rules About To Unravel Raps
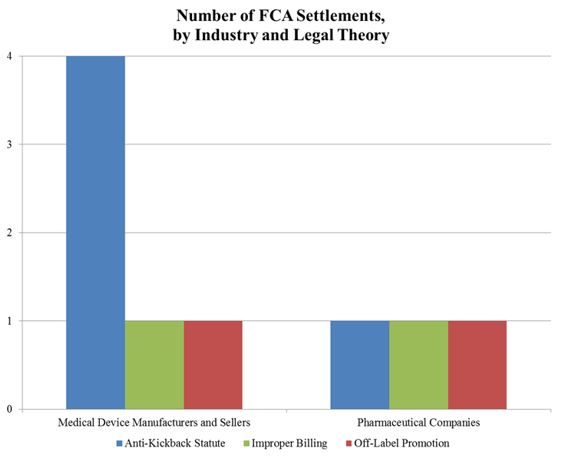 Gibson Dunn 2016 Mid Year Fda And Health Care Compliance And
Gibson Dunn 2016 Mid Year Fda And Health Care Compliance And



Belum ada Komentar untuk "29 Fda Off Label Promotion"
Posting Komentar