28 Federal Prescription Label Requirements
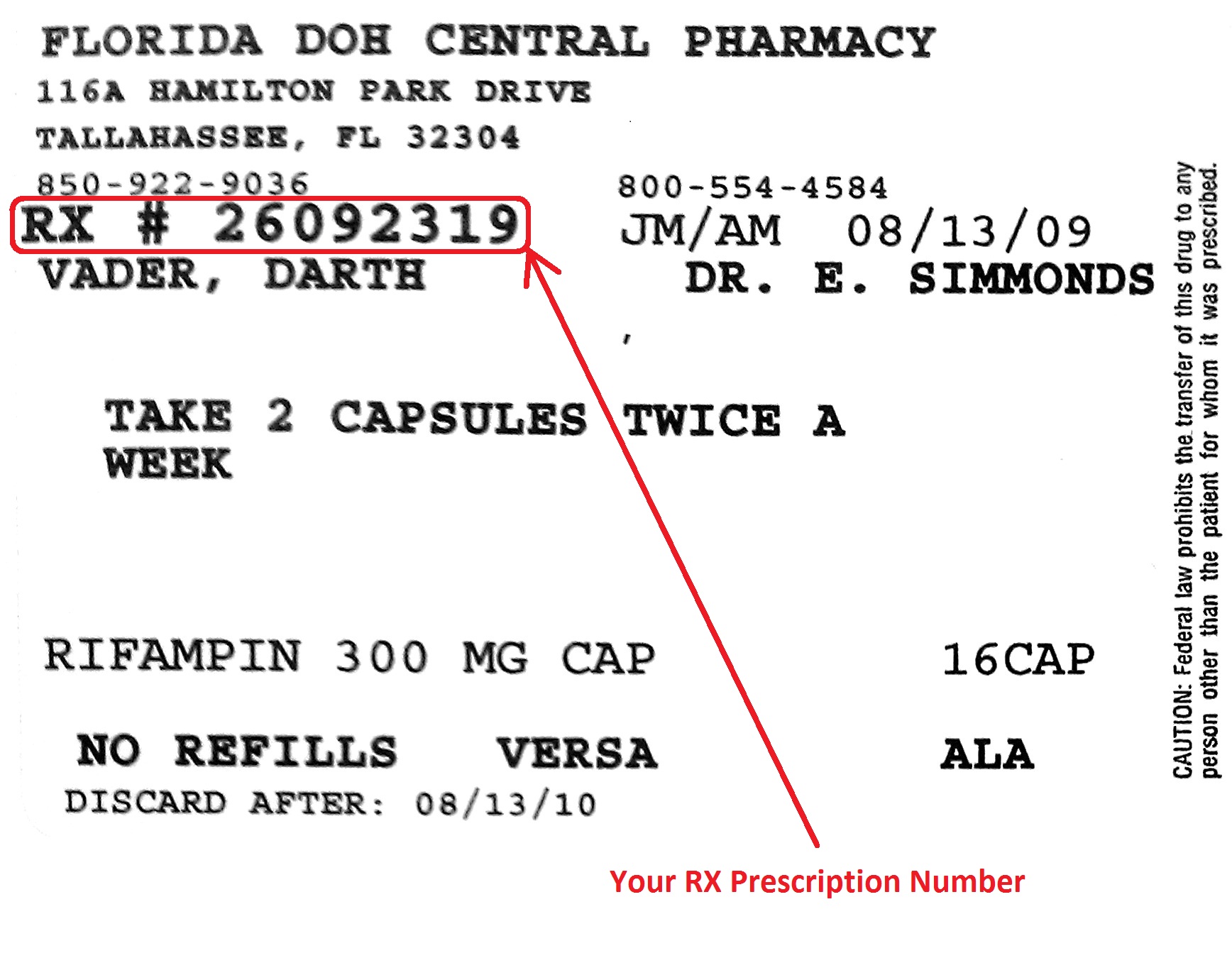
The name of the prescriber 5. The serial number of the prescription 3.
 Rx Refill Home Delivery Only Form
Rx Refill Home Delivery Only Form
20157 specific requirements on content and format of labeling for human prescription drug and biological products described in 20156b1.

federal prescription label requirements. Info that must be on a prescription bottle label. The date of the prescription date of filling or refilling 4. 20158 waiver of labeling requirements.
The goal of the physician labeling rule content and format requirements as described at 21 cfr 20156 and 20157 is to enhance the safe and effective use of prescription drug products by providing. 20156 requirements on content and format of labeling for human prescription drug and biological products. 20157 specific requirements on content and format of labeling for human prescription drug and biological products described in 20156b1.
On december 11 2003 fda published its final rule in the federal register entitled requirements for submission of labeling for human prescription drugs and biologics in electronic format 68 fr 69009. Developing standards for the conversion of paper labeling to an electronic format is a high priority for the agency. The requirements in this section apply only to prescription drug products described in 20156b1 and must be implemented according to the schedule specified in 20156c except for the requirement in paragraph c18 of this section to reprint any fda approved patient labeling at the end of prescription drug labeling or accompany the.
The name of the patient 6. 20156 requirements on content and format of labeling for human prescription drug and biological products. The pharmacy then produces a label that goes on the medication pack or bottle dispensed.
The final rule requires the content of prescription drug labeling including text tables and figures to be submitted to fda in an electronic format that the agency can. The name and address of the pharmacy 2. 20158 waiver of labeling requirements.
 Fda Draft Guidance On Responding To Unsolicited Requests For Off Labe
Fda Draft Guidance On Responding To Unsolicited Requests For Off Labe
 2 Labeling Prescriptions And Medications Basicmedical Key
2 Labeling Prescriptions And Medications Basicmedical Key
 Navigating Prescription Labels Amber Pharmacy
Navigating Prescription Labels Amber Pharmacy
 Why Amp How Some Drugs Become Over The Counter
Why Amp How Some Drugs Become Over The Counter
 Internet Pharmacies Return To Consumers The Choice Promised By
Internet Pharmacies Return To Consumers The Choice Promised By
 Cbd Labeling Guidelines And Requirements Labelvalue
Cbd Labeling Guidelines And Requirements Labelvalue
 We Asked You Answered Bath Va Medical Center
We Asked You Answered Bath Va Medical Center
 Introduction To Pharmacy Practice Ppt Video Online Download
Introduction To Pharmacy Practice Ppt Video Online Download
 Medication Orders And Labeling
Medication Orders And Labeling
 Prescription Pill Bottle Label Instructions Get Better
Prescription Pill Bottle Label Instructions Get Better
 Drug Companies Liability For The Opioid Epidemic Nejm
Drug Companies Liability For The Opioid Epidemic Nejm
 Pharmacy Technician Certification Examination Content Outline
Pharmacy Technician Certification Examination Content Outline
 What S Best For You Differences Amp Side Effects
What S Best For You Differences Amp Side Effects
 Pet Drugs Online Advocate Ppt Download
Pet Drugs Online Advocate Ppt Download
 Prescription Bottle Labels Old Age Pills Printable Rx Prescription Gag Birthday Gift Pharmacy Label Instant Download Editable Text
Prescription Bottle Labels Old Age Pills Printable Rx Prescription Gag Birthday Gift Pharmacy Label Instant Download Editable Text
 The Abcs Of Pharmacy Compliance Fda Dea And Epa
The Abcs Of Pharmacy Compliance Fda Dea And Epa
 Pharmacy Language Requirements Nationwide Language Scientific
Pharmacy Language Requirements Nationwide Language Scientific
 Bipartisan Legislation Introduced To Require Warning Labels On
Bipartisan Legislation Introduced To Require Warning Labels On
 Prescriptions Ppt Video Online Download
Prescriptions Ppt Video Online Download
 Cfr Code Of Federal Regulations Title 21
Cfr Code Of Federal Regulations Title 21
 Ppt Pharmacy Law And Ethics Powerpoint Presentation Free
Ppt Pharmacy Law And Ethics Powerpoint Presentation Free
 Pharmacist Manual Section X Dispensing Requirements Pages 1 4
Pharmacist Manual Section X Dispensing Requirements Pages 1 4
 Bobby L Rush On Twitter Quot Today I Introduced The Depression
Bobby L Rush On Twitter Quot Today I Introduced The Depression
 Annual Inventory Of Controlled Substances Wyoming State Board
Annual Inventory Of Controlled Substances Wyoming State Board
.ashx )

Belum ada Komentar untuk "28 Federal Prescription Label Requirements"
Posting Komentar