30 Cosentyx Fda Label
If a serious infection develops cosentyx until the infection resolves. Cosentyx is indicated for the treatment of adult patients with active psoriatic arthritis.
2 dosage and administration.

cosentyx fda label. The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by fda. 2 dosage and administration 21 plaque psoriasis. The drug labeling on this web site may not be the labeling on currently distributed products or identical to the labeling that is approved.
Cosentyx injection is a clear to slightly opalescent colorless to slightly yellow solution. Patients may self inject after proper training in subcutaneous injection technique using the sensoready pen or prefilled syringe and when deemed appropriate. Therefore administer the sensoready pen or prefilled syringe within 1 hour after removal from the refrigerator.
Prior to initiating treatment with cosentyx evaluate for tb. Approximately half of all 125 million patients with psoriasis suffer from scalp psoriasis24. The updated label for cosentyx in scalp psoriasis addresses an important unmet need.
With moderate to severe plaque psoriasis that involves large areas or many areas of the body and who may benefit from taking injections or pills systemic therapy or phototherapy treatment using ultraviolet or uv light alone or with systemic therapy. 51 tuberculosis tb. Scalp psoriasis can be challenging to treat with topical agents or phototherapy due to the presence of hair and other factors2.
Development history and fda approval process for cosentyx. Cosentyx secukinumab is a selective interleukin 17a il 17a inhibitor for the treatment of plaque psoriasis ankylosing spondylitis and psoriatic arthritis. Cosentyx secukinumab is a prescription medicine used to treat adults.
Cosentyx is indicated for the treatment of adult patients with active ankylosing spondylitis. 13 ankylosing spondylitis. Cosentyx is indicated for the treatment of adult patients with active psoriatic arthri tis.
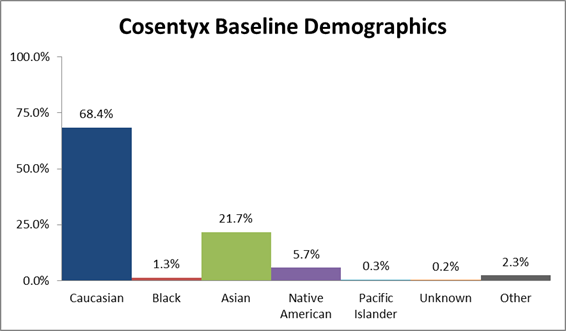
Cosentyx is indicated for the treatment of adult patients with active ankylosing spondylitis. A total of 3430 plaque psoriasis subjects were treated with cosentyx in controlled and uncontrolled clinical trials. Do not use if the liquid contains visible particles is discolored or cloudy.
Caution should be exercised when considering the use of cosentyx in patients with a chronic infection or a history of recurrent infection. Of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Cosentyx is intended for use under the guidance and supervision of a physician.
Cosentyx does not contain preservatives.
 Arthritis Psoriasis Medications Drugs Deemed Safe Get Fda Warning
Arthritis Psoriasis Medications Drugs Deemed Safe Get Fda Warning
 Scalp Psoriasis Data Added To Secukinumab Labeling Dermatology
Scalp Psoriasis Data Added To Secukinumab Labeling Dermatology
 Cosentyx Entresto Again Drive Growth At Novartis But What About
Cosentyx Entresto Again Drive Growth At Novartis But What About
 Cosentyx Secukinumab For Treatment Of Plaque Psoriasis
Cosentyx Secukinumab For Treatment Of Plaque Psoriasis
 Novartis Obtains European Approval For Cosentyx To Treat Psoriasis
Novartis Obtains European Approval For Cosentyx To Treat Psoriasis
 Fda Approves Label Update For Secukinumab In Ankylosing
Fda Approves Label Update For Secukinumab In Ankylosing
 These Highlights Do Not Include All The Information Needed To Use
These Highlights Do Not Include All The Information Needed To Use
 Full Text Secukinumab For Rheumatology Development And Its
Full Text Secukinumab For Rheumatology Development And Its
 Novartis Cosentyx Gets Fda Approval For Arthritis Treatment Epm
Novartis Cosentyx Gets Fda Approval For Arthritis Treatment Epm
 Novartis Cosentyx Scores Again In Phase 3 Strengthens Approval
Novartis Cosentyx Scores Again In Phase 3 Strengthens Approval
 Fda Approves Label Update For Secukinumab Hcplive
Fda Approves Label Update For Secukinumab Hcplive
 Retina Today Systemic Options For Treating Uveitis October 2018
Retina Today Systemic Options For Treating Uveitis October 2018
 Clinical Trials Clinical Trial Spotlight Patient
Clinical Trials Clinical Trial Spotlight Patient
 These Highlights Do Not Include All The Information Needed To Use
These Highlights Do Not Include All The Information Needed To Use
 Novartis Psoriasis Drug Cosentyx Positive In Scalp Study Nasdaq
Novartis Psoriasis Drug Cosentyx Positive In Scalp Study Nasdaq
 Novartis Gets Fda Approval For Cosentyx Label Update
Novartis Gets Fda Approval For Cosentyx Label Update
 Abbvie S Risankizumab Has Uptake Caveats Despite Anticipated Approvals
Abbvie S Risankizumab Has Uptake Caveats Despite Anticipated Approvals
 Janssen Announces Fda Approval Of Stelara For Plaque Psoriasis
Janssen Announces Fda Approval Of Stelara For Plaque Psoriasis
 Fda Approves Treatment For Non Radiographic Spondyloarthritis
Fda Approves Treatment For Non Radiographic Spondyloarthritis
 Cosentyx Gains Fda Approval For Scalp Psoriasis Pdf Archive
Cosentyx Gains Fda Approval For Scalp Psoriasis Pdf Archive
 Novartis Receives Fda Approval For Cosentyx Label Update To
Novartis Receives Fda Approval For Cosentyx Label Update To
 2019 Q4 And Full Year Results Presentation Amp Transcript Novartis
2019 Q4 And Full Year Results Presentation Amp Transcript Novartis
 Janssen Announces U S Fda Approval Of Tremfya Guselkumab For
Janssen Announces U S Fda Approval Of Tremfya Guselkumab For
 Ndc 0078 0639 Cosentyx Secukinumab
Ndc 0078 0639 Cosentyx Secukinumab
 Cosentyx Secukinumab Injection Uses Dosage Side Effects
Cosentyx Secukinumab Injection Uses Dosage Side Effects

 Fda Approval Of The First Nr Axspa Biologics Projecting Regulatory
Fda Approval Of The First Nr Axspa Biologics Projecting Regulatory


Belum ada Komentar untuk "30 Cosentyx Fda Label"
Posting Komentar