29 Darzalex Fda Label
See full prescribing information for darzalex. The darzalex dosing schedule in table 2 is for combination therapy with bortezomib melphalan and prednisone 6 week cycle regimen for patients with newly diagnosed multiple myeloma ineligible for asct.
 Hematologic Cancer Targets Cancer Network
Hematologic Cancer Targets Cancer Network
Infusion rates and management of infusion reactions administer darzalex infusion intravenously at the infusion rate described below in table 4.

darzalex fda label. Darzalex is for single use only. Darzalex daratumumab injection for intravenous use. 71 effects of daratumumab on laboratory tests 8 use in specific populations.
062019 71 effects of daratumumab on laboratory tests 8 use in specific populations 81 pregnancy. On november 21 2016 the us. Fda approves darzalex daratumumab in combination with pomalidomide and dexamethasone for relapsed or refractory multiple myeloma nov 21 2016 fda approves darzalex daratumumab in combination with two standard of care regimens for the treatment of patients with multiple myeloma who have received at least one prior therapy.
Darzalex safely and effectively. See full prescribing information for darzalex. 24 preparation for administration.
See 17 for patient counseling information and fda approved patient labeling. Darzalex safely and effectively. For dosing instructions of combination agents administered with darzalex see clinical studies 14 and manufacturers prescribing information.
If a planned dose of darzalex is missed administer the dose as soon as possible and adjust the dosing schedule accordingly maintaining the treatment interval. On june 27 2019 the food and drug administration approved daratumumab darzalex janssen biotech. Darzalex daratumumab injection for intravenous use.
See 17 for patient counseling information and fda approved patient labeling. Calculate the dose mg total volume ml of solution required and the darzalex number of darzalex vials needed based on patient actual body weight. Prepare the solution for infusion using aseptic technique as follows.
Fda approves daratumumab for multiple myeloma ineligible for autologous stem cell transplant. Darzalex see manufacturers prescribing information. See full prescribing information for darzalex.
71 effects of daratumumab on laboratory tests 8 use in specific populations 81 pregnancy 82. On september 26 2019 the food and drug administration approved daratumumab darzalex janssen for adult patients with multiple myeloma in combination with bortezomib thalidomide and. Darzalex safely and effectively.
Food and drug administration approved daratumumab darzalex janssen biotech inc in combination with lenalidomide and dexamethasone or bortezomib and. Darzalex daratumumab injection for intravenous use. See 17 for patient counseling information and fda approved patient labeling.
 Fda Expands Daratumumab S Indication To Frontline Myeloma
Fda Expands Daratumumab S Indication To Frontline Myeloma
 Fda S Ok Sought For Darzalex Combo To Treat Certain Myeloma Patients
Fda S Ok Sought For Darzalex Combo To Treat Certain Myeloma Patients
 Fda Approval Of Darzalex International Myeloma Foundation
Fda Approval Of Darzalex International Myeloma Foundation
 Janssen Seeks Ema Approval For Novel Subcutaneous Formulation Of
Janssen Seeks Ema Approval For Novel Subcutaneous Formulation Of

 Karyopharm Comes To Boston For The Springtime Evaluate
Karyopharm Comes To Boston For The Springtime Evaluate
 Darzalex As Multiple Myeloma Combo Therapy Under Final Fda Review
Darzalex As Multiple Myeloma Combo Therapy Under Final Fda Review
 Fda Reviews Sanofi S Multiple Myeloma Drug Isatuximab
Fda Reviews Sanofi S Multiple Myeloma Drug Isatuximab
 Phase Iii Darzalex Results Suggest Drug Could Have Immediate
Phase Iii Darzalex Results Suggest Drug Could Have Immediate
 Janssen Announces Darzalex Daratumumab U S Fda Approval For
Janssen Announces Darzalex Daratumumab U S Fda Approval For
 Fda Approves Mace Label Claim For Lilly S Trulicity
Fda Approves Mace Label Claim For Lilly S Trulicity
Genmab A S Genmab Announces Approval Of Darzalex R Daratumumab
 I3 Health News Amp Perspectives I3 Health News Daratumumab Fda
I3 Health News Amp Perspectives I3 Health News Daratumumab Fda
 I3 Health News Amp Perspectives I3 Health News New Daratumumab
I3 Health News Amp Perspectives I3 Health News New Daratumumab
 Darzalex Combination Therapy Approved In Usa
Darzalex Combination Therapy Approved In Usa
 Fda Approves Daratumumab Plus Vmp For Transplant Ineligible
Fda Approves Daratumumab Plus Vmp For Transplant Ineligible
 Fda Approves Novel Therapy For Relapsed Refractory Multiple Myeloma
Fda Approves Novel Therapy For Relapsed Refractory Multiple Myeloma
 Amgen And Ucb S Evenity Finally Approved But With Label Warnings
Amgen And Ucb S Evenity Finally Approved But With Label Warnings
 Multiple Myeloma Can Be Treated With Darzalex As Split Dose Over
Multiple Myeloma Can Be Treated With Darzalex As Split Dose Over
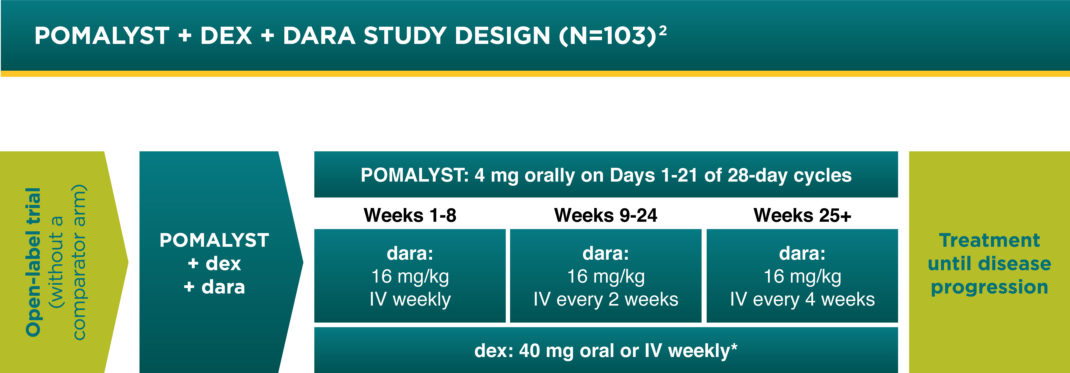 Pomalyst Dex Dara Trial Design Pomalyst Pomalidomide
Pomalyst Dex Dara Trial Design Pomalyst Pomalidomide

 Fda Approves Darzalex For Newly Diagnosed Mm Clinical Oncology News
Fda Approves Darzalex For Newly Diagnosed Mm Clinical Oncology News
 Fda Approves Daratumumab For Multiple Myeloma Ineligible For
Fda Approves Daratumumab For Multiple Myeloma Ineligible For
 Darzalex Could Boost Jnj S Revenue Growth In 2018 Market Realist
Darzalex Could Boost Jnj S Revenue Growth In 2018 Market Realist
 Genmab J Amp J Score Another Quickie Darzalex Approval Fiercebiotech
Genmab J Amp J Score Another Quickie Darzalex Approval Fiercebiotech
 New Indication For Darzalex Approved By Fda Managed Healthcare
New Indication For Darzalex Approved By Fda Managed Healthcare


Belum ada Komentar untuk "29 Darzalex Fda Label"
Posting Komentar