28 Carboplatin Fda Label
Carboplatin injection multidose vials maintain microbial chemical and physical stability for up to 14 days at 25c following multiple needle entries. Each ml contains 10 mg carboplatin and water for injection usp.
 Fda Approves Atezolizumab With Chemotherapy And Bevacizumab For
Fda Approves Atezolizumab With Chemotherapy And Bevacizumab For
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
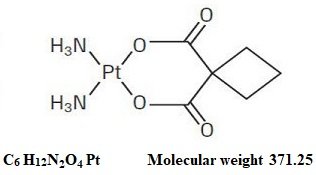
carboplatin fda label. It is used with other chemotherapy as first line treatment. Ovarian cancer that is advanced. Paraplatin carboplatin aqueous solution injection is contraindicated in patients with a history of severe allergic reactions to cisplatin or other platinum containing compounds.
May enhance the ototoxic effect of carboplatin. Carboplatin injection is supplied as a sterile pyrogen free 10 mgml aqueous solution of carboplatin usp. Carboplatin injection is supplied as a sterile pyrogen free aqueous solution available in 50 mg5 ml 150 mg15 ml 450 mg45 ml or 600 mg60 ml multi dose vials containing 10 mgml of carboplatin for administration by intravenous infusion.
Fda label information for this drug is available at dailymed. Especially with higher doses of carboplatin. Multidose vials are stable for up to 14 or 15 days after opening when stored at 25c 77f following multiple needle entries refer to specific product labeling for stability information.
All of the platinum in the 24 hour urine is present as carboplatin. Carboplatin usp is a platinum coordination compound. Each ml contains 10 mg carboplatin usp 10 mg mannitol and water for injection usp.
Carboplatin is approved to be used alone or with other drugs to treat. The major route of elimination of carboplatin is renal excretion. Patients with creatinine clearances of approximately 60 mlmin or greater excrete 65 of the dose in the urine within 12 hours and 71 of the dose within 24 hours.
Due to inconsistencies between the drug labels on dailymed and the pill images provided by rximage we no longer display the rximage pill images associated with drug labels. We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels. It is used alone as palliative treatment for disease that has recurred come back after earlier chemotherapy.
Solutions for infusion should be discarded 8 hours after preparation. Paraplatin should not be employed in patients with severe bone marrow depression or significant bleeding.
 Carboplatin App Pharmaceuticals Llc Fda Package Insert Page 8
Carboplatin App Pharmaceuticals Llc Fda Package Insert Page 8
 New Indications Approved By The Fda In 2018 For Oncology Drugs
New Indications Approved By The Fda In 2018 For Oncology Drugs
 Recent Fda News Advancing Treatments In Oncology Article
Recent Fda News Advancing Treatments In Oncology Article
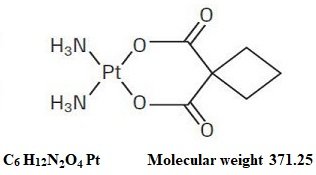 Carboplatin Fda Prescribing Information Side Effects And Uses
Carboplatin Fda Prescribing Information Side Effects And Uses
 Checkpoint Inhibitor Use Changed For Bladder Cancer National
Checkpoint Inhibitor Use Changed For Bladder Cancer National
 Fda Expands Keytruda Approval For First Line Nsclc Treatment
Fda Expands Keytruda Approval For First Line Nsclc Treatment
 Carboplatin Injection Carboplatin Aqueous Solution
Carboplatin Injection Carboplatin Aqueous Solution
 I3 Health News Amp Perspectives I3 Health News Atezolizumab Plus
I3 Health News Amp Perspectives I3 Health News Atezolizumab Plus
 Fda Approves Expanded Label For Merck S Keytruda In Patients With
Fda Approves Expanded Label For Merck S Keytruda In Patients With
 Pdf Carboplatin Induced Hematotoxicity Among Patients With Non
Pdf Carboplatin Induced Hematotoxicity Among Patients With Non
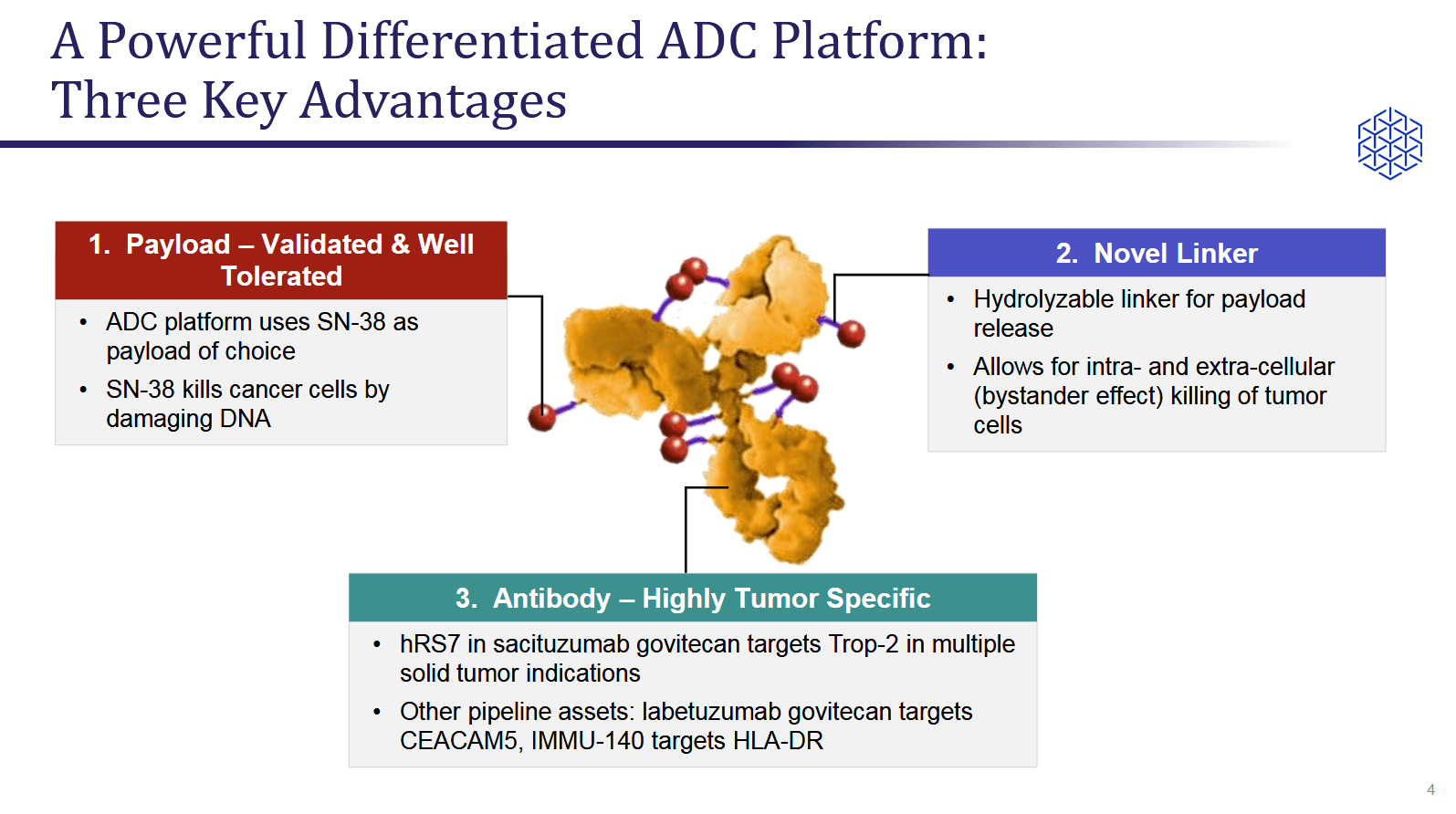 Immunomedics Competitor Threatens To Leapfrog Approval With
Immunomedics Competitor Threatens To Leapfrog Approval With
 Fda Approves Genentech S Tecentriq Plus Chemotherapy Abraxane And
Fda Approves Genentech S Tecentriq Plus Chemotherapy Abraxane And
 Biosimilar Cancer Treatment Ontruzant Gets Fda Approval Cancer
Biosimilar Cancer Treatment Ontruzant Gets Fda Approval Cancer
Patent Docs Food And Drug Administration
 Health News Roundup Roche Wins Fda Approval For Immunotherapy
Health News Roundup Roche Wins Fda Approval For Immunotherapy
 Roche S Tecentriq Atezolizumab Chemotherapy Carboplatin And
Roche S Tecentriq Atezolizumab Chemotherapy Carboplatin And
 Lung Cancer Immunotherapy Gains Fda Approval Vax Before Cancer
Lung Cancer Immunotherapy Gains Fda Approval Vax Before Cancer
 Frontline Pembrolizumab Combo Approved By Fda For Nsclc
Frontline Pembrolizumab Combo Approved By Fda For Nsclc
 In Second Line Treatment Of Advanced Nsclc Adding Carboplatin To
In Second Line Treatment Of Advanced Nsclc Adding Carboplatin To
 Ndc 50742 447 Carboplatin Carboplatin
Ndc 50742 447 Carboplatin Carboplatin
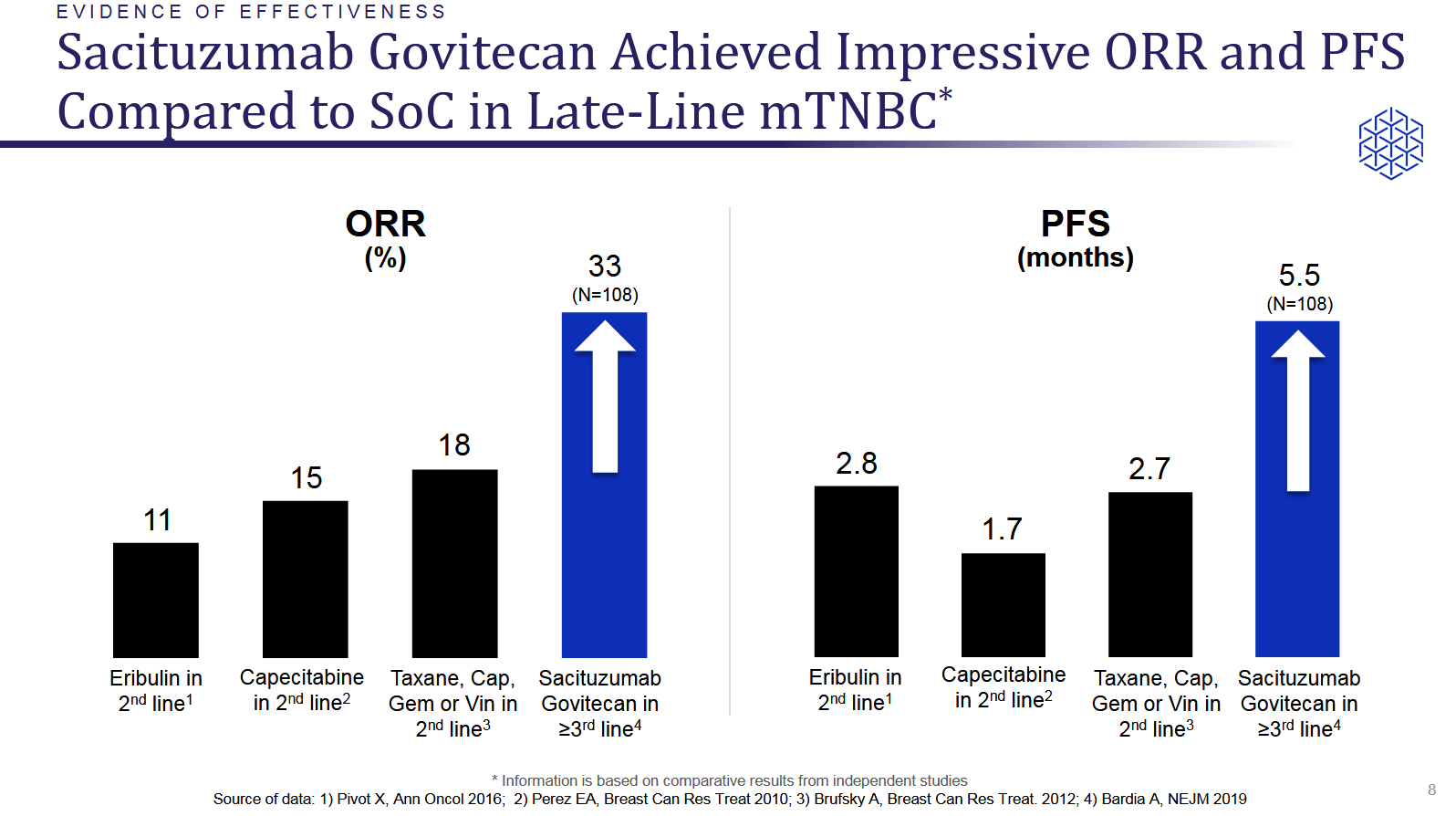 Immunomedics Competitor Threatens To Leapfrog Approval With
Immunomedics Competitor Threatens To Leapfrog Approval With

 Carboplatin Auromedics Pharma Llc Fda Package Insert Page 6
Carboplatin Auromedics Pharma Llc Fda Package Insert Page 6
 Janssen Seeks Expanded Fda Approval Of Erleada For Metastatic Cspc
Janssen Seeks Expanded Fda Approval Of Erleada For Metastatic Cspc



Belum ada Komentar untuk "28 Carboplatin Fda Label"
Posting Komentar